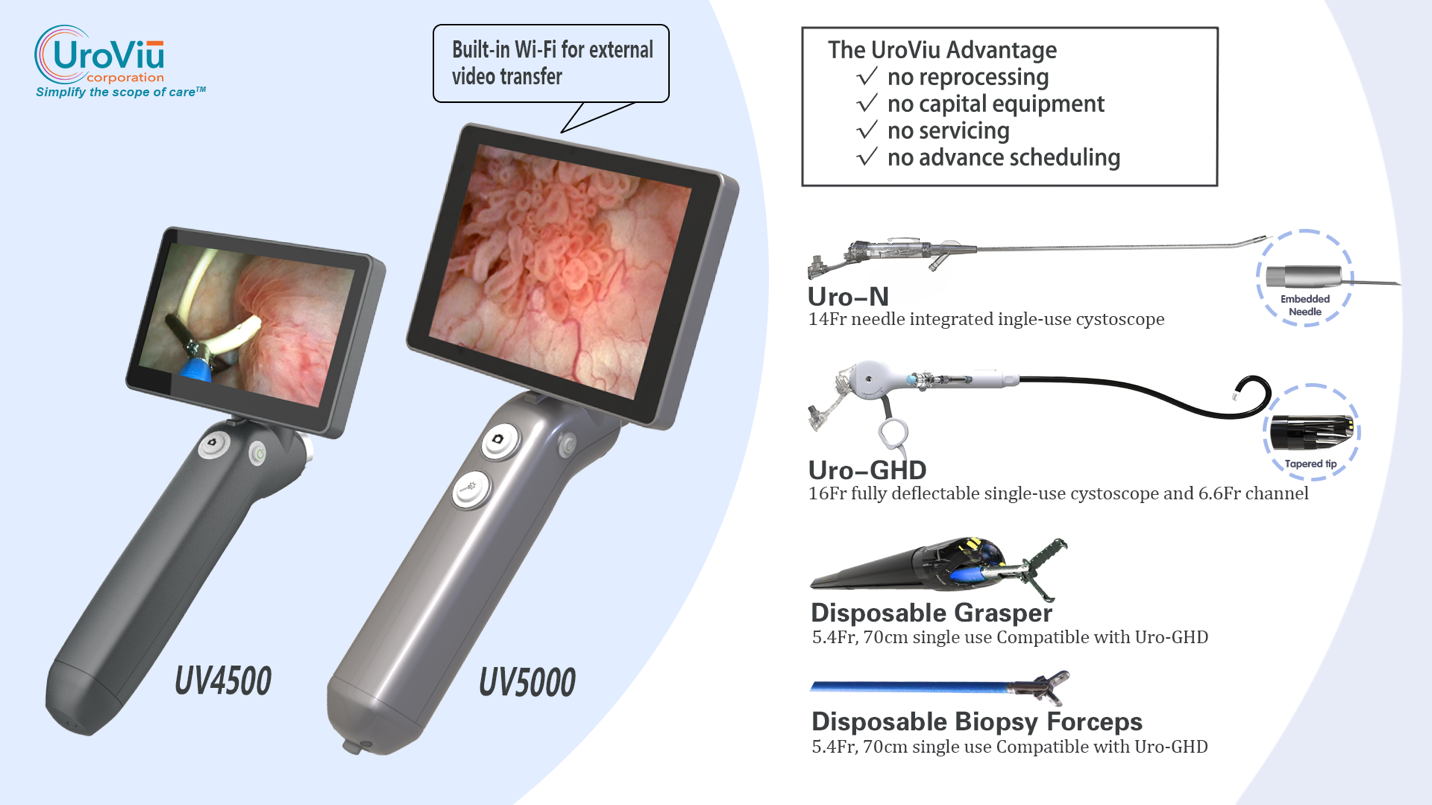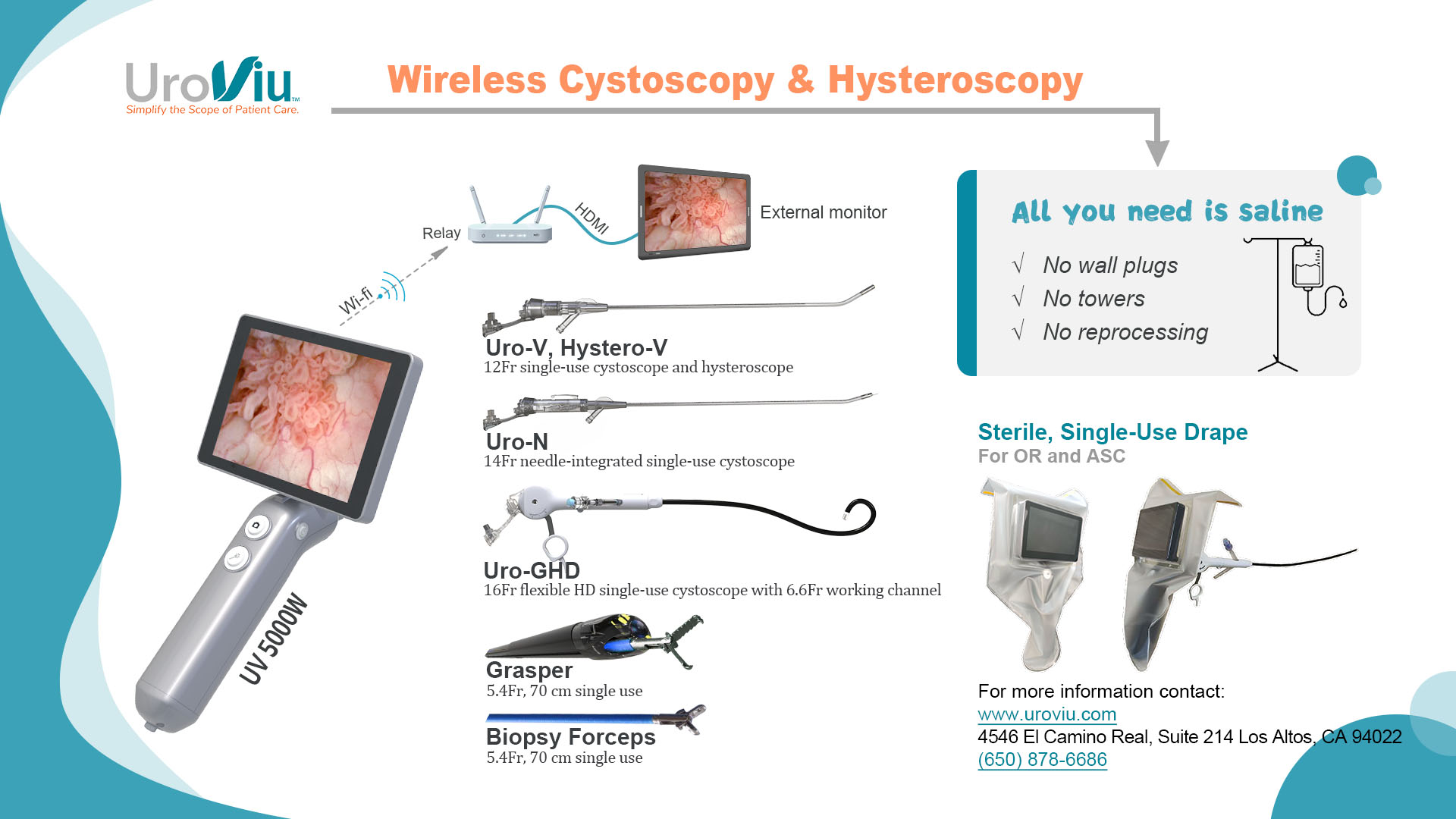
UroViu Receives CE certification of its cystoscopic product portfolio
UroViu Corporation announced today that it has received CE certification for its cystoscopic product portfolio. This includes the reusable UV4500 Handle and the disposable Hystero-V, Uro-V, and Uro-GHD endoscopes. “This CE certification marks an important milestone for UroViu and for patients worldwide,” said Bruce OuYang, Ph.D., CEO. “It enables us to bring the most versatile, portable and environmentally friendly portfolio of cystoscopes to patients in any region that accepts the CE Marking.” This certification also lays a foundation for fast-track approval of future new releases or upgrades within the same product line. The UroViu portfolio has been widely adopted across various segments of the U.S. Urology and UroGynecology market, including private practices, Ambulatory Surgical Centers (ASCs), academic institutions, VA, and DOD healthcare facilities. UroViu products offer several unique features: Self Contained, Cordless, Untethered Design: This design minimizes environmental impact and the carbon footprint of disposable products by eliminating the need