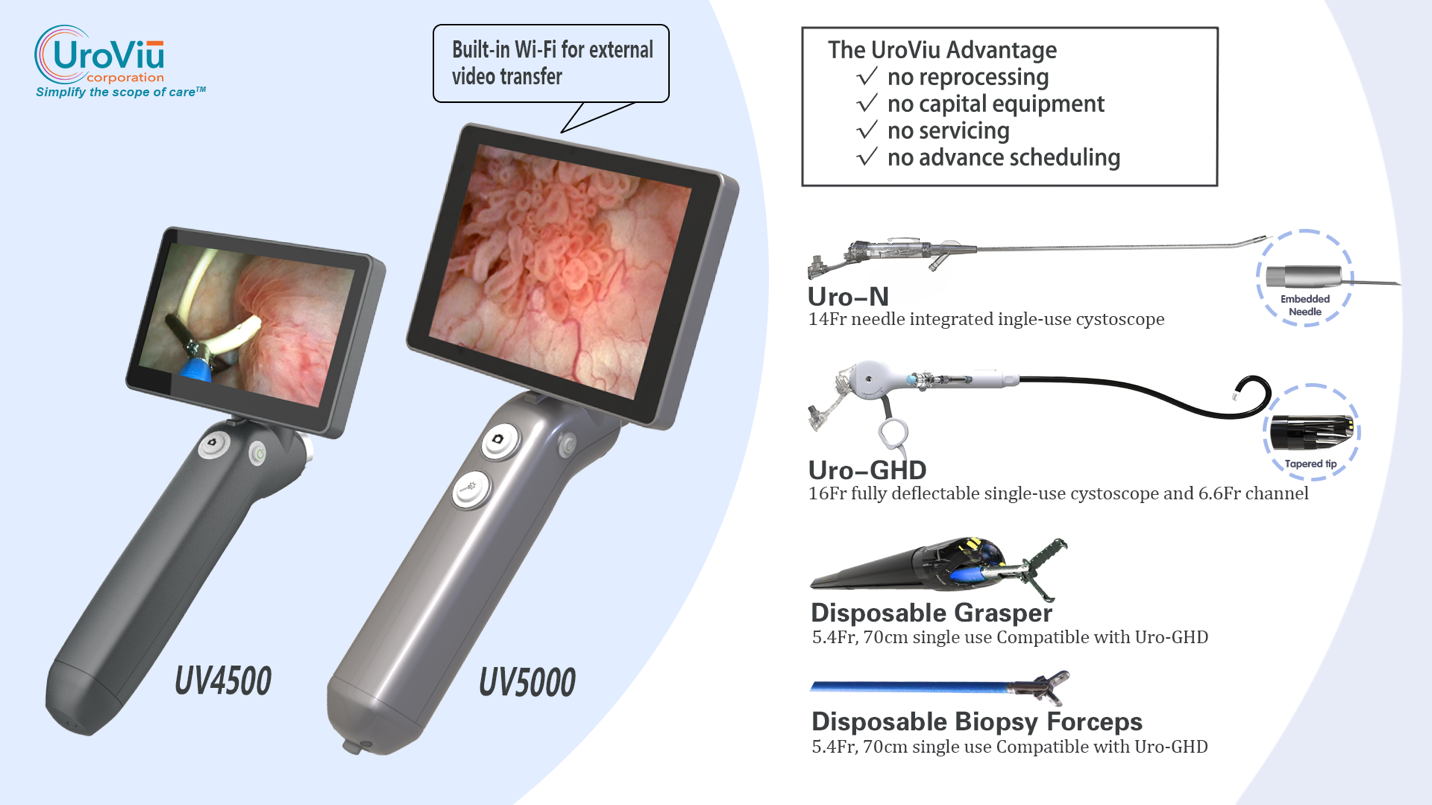
UroViu Receives FDA 510(k) Clearance for Its UV5000 Advanced Endoscope Platform
UroViu Corporation is pleased to announce that the U.S. Food and Drug Administration (FDA) has granted 510(k) clearance for its UV5000 cordless, single-use endoscope platform. The UV5000 replaces the current UV4500 on the market, offering a faster processor, longer battery life, and brighter HD visualization directly within the clinician’s line of sight. It is compatible with all of UroViu’s single-use endoscope cannulas, graspers, and biopsy forceps. As a companion to the UV5000, UroViu has submitted a 510(k) application for a model, the UV5000W, that has built-in Wi-Fi connectivity to external displays. Bruce OuYang, Ph.D., CEO, commented, “UroViu strives to achieve a win-win situation for patients and care providers and has gained adoption across all segments of the market, including private practices, ASCs, academic institutions, VA, and DOD healthcare facilities, benefiting from the following unique features: Cordless, untethered design: This minimizes the environmental impact and carbon footprint of disposable products by